Emission of odors from ponds and lagoons occurs due to aerobic and anaerobic decomposition of organic solids to release hydrogen sulfide, ammonia, and other organic by-products. Aerobic biodegradation occurs near the water-air interface, where diffusion of oxygen occurs, while anaerobic degradation prevails mainly near the bottom of the pond or lagoon, where there is low or no dissolved oxygen in the water.
The biodegradation reactions that occur to release hydrogen sulfide and ammonia can be written as follows:
Organic matter + SO4 2- — S 2-+H2O+CO2
S 2- + 2H+ ————– H2S
4(CH3)3N+H2O ———9CH4+3CO2+6H2O+4NH3 (organic matter)
Both hydrogen sulfide and ammonia gases have strong odors with high detection thresholds. The gas bubbles are produced by aerobic degradation of organics into carbon dioxide and water as well as from the generation of methane by anaerobic decomposition of organic matter.
Dissolution of oxygen in wastewater is essential for achieving several benefits which includes:
- Improved biodegradation of organics resulting in lower release of BOD in the exit water;
- Lesser accumulation of organic solids in the pond, resulting in increased capacity and eliminating periodic dredging of solid sediments;
- Elimination of odor emissions from the pond (hydrogen sulfide, ammonia, etc.);
- Elimination of foam formation on the surface, due to surfactants; and
- Possibility of water recycling and reuse after treatment in the pond, due to improved water quality.
Generally, lack of dissolved oxygen in ponds and lagoons results in emission of odorous gases and in some cases formation of foam on the surface. Modifying the mixing patterns in the pond or lagoon requires major changes in the flow system, and use of aerators is expensive both from an investment and operating point of view, since it requires installation and operation of air blowers. Further, a majority of the air sparged into a pond or lagoon leaves the water surface and does not result in high dissolved oxygen levels in areas of poor liquid mixing.
Ensuring proper liquid mixing in the pond or lagoon is essential for achieving better oxygen utilization, improved digestion of organic solids, and elimination of emission of odorous gases from the wastewater.
The non-active biocatalysts as OrTec are capable of delivering accelerated biotreatment for both aerobic and anaerobic cultures. The use of them can be a cost-effective solution for control of generation of hydrogen sulfide and/or ammonia in anaerobic digesters, aeration basins, ponds, lagoons, sewer lines and other anoxic systems. , etc.
Experimental studies were conducted to test the efficacy of non-active biocatalysts in preventing emission of odors from wastewater:
(a) Sparging with air;
(b) Sparging with nitrogen gas; and
(c) No sparging of gases.
Odor emissions were quantified by a human odor panel. The human nose is the only effective method to monitor the presence of odorous gases, since most analytical instruments are not as sensitive. Further, the human nose can distinguish between “new odors” as compared to “old odors”, which is not possible with any analytical instrument. The gas samples were collected in 10-liter tedlar bags and analyzed by a human odor panel.
The tedlar bags commonly used for industrial air sampling purposes were obtained from Sigma-Aldrich Company. A Supelco sampling apparatus equipped with a small sampling pump and a rotameter was used to collect the air samples. A flexible ¼” tubing was connected from the sample port through the ¼” fitting in the Supelco apparatus, to an empty tedlar bag placed with nozzle facing up in the Supelco sampling box. The box was closed tight with the tedlar bag in place, and the sampling pump was turned on to create a vacuum in the headspace. The negative headspace pressure allowed the tedlar bag to inflate, withdrawing air from the sample ports at an adjustable rate of 0-5 liters per minute (lpm). A level strip circuit breaker switch located at the top of the apparatus, turned off the sampling pump automatically when the bag was fully inflated. The Supelco box was opened, tedlar bag valve was closed immediately by pulling it tight, labeled, and removed from the apparatus. Each bag was labeled using a unique identification method that indicated the sampling date and location. The time of sample collection was also noted on each label. Each bag was “pre-conditioned” by partially inflating the bag and emptying it by pressing against the chest, prior to collecting each air sample. The pre-conditioned tedlar bags were then allowed to fully inflate to obtain a representative air sample of the receptor location
The panel members were each provided with a response sheet and were instructed to rank the responses of odors inhaled from a scale of 1 to 10, one being the least offensive odor and 10 being the most offensive odor.
In order to further reduce human response bias, carbon filtered air samples were anonymously included and numbered sequentially with the odor samples. Additionally, a certain number of samples were analyzed in duplicate by re-numbering the bags. The purpose of analysis of carbon filtered air samples and duplicate odor samples was to evaluate variability in the panelists’ qualitative nature of ranking the odors and determine the data reproducibility factor.
Detection threshold is the lowest concentration of an odor in the air sample at which the human olfactory response is detected, as determined by the best-estimate criterion. Recognition threshold is the lowest concentration of the odor at which the human olafactory response can recognize the odor, as also determined by the best-estimate criterion. This criterion is defined as the geometric mean of the two adjacent concentrations, between which the olfactory response is registered.
Odors are difficult to perceive by human receptors when the odor levels are at or barely above the threshold value. Typically, the recognition threshold, expressed in the form of concentration, exceeds the detection threshold by a factor of 2 to 10. However, the threshold values can also be expressed as a volume ratio, referred to as dilution-to-threshold ratio, and is defined in terms of the following dimensionless quantity:
Dilution-to-threshold (Z) = V/v (1)
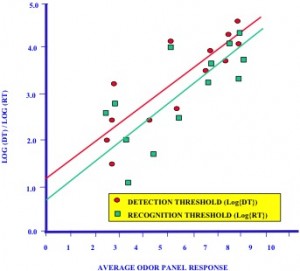
where V is the volume of odor diluted to threshold level, and v is the volume of the odorous sample. The dilution-to-threshold value increases with odor concentration. Calibration data was initially obtained to convert the odor panel ratings to detection and recognition thresholds. Figure 1 is a plot of log (DT) or log (RT) versus average response of the odor panel, where DT is the detection dilution-to-threshold value and RT is the recognition dilution-to-threshold value. A linear correlation was derived from the experimental data, and the following correlations were obtained:
log (DT) = 1.2 + 0.416* r R2 = 0.82 (2)
log (RT) = 0.72 + 0.44*r R2 = 0.74 (3)
where DT is the detection dilution-to-threshold value, RT is the recognition dilution-to-threshold value and r is the average odor panel response. The linear correlations allow the average odor panel response to be converted to DT and RT values.
Plot of Detection and Recognition Thresholds versus the Average Response of an Odor Panel.
Odor Panel Results
Odor levels in the exit gas was analyzed by a human odor panel. The response of the odor panel was averaged and converted to Detection Threshold values using the correlation given in Figure 1. Detection Threshold quantifies the level of odors present in the gas. Results of this analysis are shown in Table 1. Odors generated were significantly greater with nitrogen gas than with air, and this is understandable, since using nitrogen gas would allow sulfate reducing organisms to grow in the wastewater, resulting in the formation of sulfide. Under aerobic conditions, ammonia would be formed, if the wastewater had significant quantities of nitrogen compounds.
The gas leaving the experimental vial was collected in a tedlar bag and subjected to a human odor panel evaluation. Since 10 Liter tedlar bags were used to collect the sample gas, each sample was sparged over a 6 hour time period. The following average values of Detection Thresholds were obtained.
Table 1. Average Values of Detection Thresholds Using Air and Nitrogen Gas with and without Biocatalyst.
|
Non-Active Biocatalyst Not Added to Sample |
Air Sparged into Sample |
Nitrogen Gas Sparged into Sample |
||||
|
3,240+ 250 (air)
5,980+ 345 with Nitrogen gas
|
0.01 ppm |
0.03 ppm |
0.1 ppm |
0.01 ppm |
0.03 ppm |
0.1 ppm |
|
1,840 +172 |
786 +82 |
120 +28 |
2,650 +275
|
1,670 +172 |
134 +36 |
|
As a reference, carbon filtered air, analyzed by the same odor panel gave an average Detection Threshold value of 127+32, even though carbon filtered air has no odors present. Since smell is a subjective sensation, odor panels often give variable results. However, it can be concluded that Detection Thresholds in the range of 100-160 have no detectable odors present. Further, in any operating system, any gases exiting the wastewater would be further diluted with ambient air, thereby reducing their Detection Threshold values.
When the inlet air was not sparged in the liquid, the measured Detection Threshold was 2672 + 234 without non-active biocatalyst present. With non-active biocatalyst added to the wastewater sample, the results were: 1656 + 172 with 0.01 ppm non-active biocatalyst concentration, 678 + 89 with 0.03 ppm non-active biocatalyst concentration, and 92 + 11 with 0.1 ppm non-active biocatalyst concentration. Clearly, when gases were not sparged in the liquid sample, mass transfer from the liquid bulk to the liquid-gas interface controlled the emission of the odors. Results in this case were intermediate between air sparging and nitrogen gas sparging, indicating that part of the sample was aerobic near the air-liquid interface, and the rest of the sample was anoxic to some extent.