Anaerobic degradation of organics and solids is known to produce hydrogen sulfide, ammonia and methane gases. Occurrence of anoxic conditions due to maldistribution of dissolved oxygen in aqueous systems is a major problem in wastewater treatment plants.
A new solution contemplates the use of Non-Active Biocatalysts as OrTec to improve biodegradation rates of both aerobic and anaerobic bacterial cultures and to prevent the reduction of sulfate and/or nitrate to hydrogen sulfide and ammonia, respectively.
Major factors in maintaining adequate dissolved oxygen concentrations are:
- Low solubility of dissolved oxygen from air at atmospheric pressure and ambient temperature;
- Poor mass transfer from the air phase to the aqueous phase, unless the air is distributed in the form of fine bubbles;
- Low residence times of air bubbles in the liquid phase due to large differences between the air and water density;
- Inadequate mixing of the liquid which limits the distribution of dissolved oxygen;
- Bypassing of the air and water in the treatment system;
- Inadequate distribution of air bubbles in dead zones, such as in corners of rectangular tanks, near tank walls, etc.
- Decrease in oxygen solubility due to decreased temperature at colder temperatures, such as in wintertime in the northeast U.S.; and
- Coalescence of small air bubbles into large bubbles with limited surface area.
Attempts have been made to improve introduction of dissolved oxygen in wastewater by using fine bubble membrane diffusers with several rows of diffusers in aeration tanks. Also, use of smaller but larger number of aeration basins to prevent bypassing of water and ensuring good distribution of the influent water into several parallel rows of basins, are strategies to ensure good mixing conditions. However, considerable energy and operating costs are expended in attempting to diffuse large volumes of air into the wastewater liquid. In spite of all these design improvements, due to rapid consumption of oxygen by aerobic bacteria in aeration basins, when high BOD (Biological Oxygen Demand) is present in wastewater, oxygen limitations arise, resulting in the development of anoxic zones.
Development of anoxic conditions is especially acute in sewer lines and anaerobic reactors, where there is no aeration. Due to large residence times resulting in considerable aging of the biosolids, significant amounts of hydrogen sulfide can be generated.
Hydrogen sulfide is produced by the anoxic decomposition of sulfate and ammonia by the anaerobic degradation of organic-nitrogen, present in the wastewater. Hydrogen sulfide can also be produced in anoxic zones in aeration tanks, primary and secondary clarifiers and in sludge lines.
Organic matter + SO42- ——- S 2- + H2O + CO2
S 2- + 2H+ ——————– H2S
4(CH3)3N + H2O ————— 9CH4 + 3CO2 + 6H2O + 4NH3
Traditional methods to treat problems associated with hydrogen sulfide and/or ammonia in wastewater treatment plants have involved three main approaches:
- Improvements in dissolved oxygen levels by increasing the rate of air sparging, installing more air diffusers, modifying flow hydraulics, etc.;
- Using additives, which either complexes sulfides in the water, prevent the growth of sulfate reducers and/or nitrate reducers or provide alternate electron acceptors than sulfate and/or nitrate; and
- Collection of air from the headspace of tanks and treatment using chemical oxidation scrubbers (hypochlorite and sodium hydroxide, for example) or biofilters (compost or synthetic media biotrickling designs).
As mentioned earlier, ensuring proper distribution of dissolved oxygen in wastewater is energy intensive and difficult due to large flow rates, limited residence times of sparged gas bubbles, low oxygen solubility, and rapid consumption of oxygen by localized active aerobic bacteria. Additives have been used successfully in many plants; however, major problems associated with additives are: (a) high cost of additive; (b) inability to disperse the additive effectively in the wastewater; and (c) effective transfer of the hydrogen sulfide and/or ammonia into the air, resulting in odor emissions, when the wastewater is mixed rapidly with the additive, using liquid mixing systems. In many cases, collection and treatment of air is difficult from open aeration tanks, clarifiers, headworks, etc. and installation of flexible covers is expensive, and requires confined space entry issues when water sampling, conducting repairs, etc. Scrubbers and Biofilters can be effective when emissions from enclosed systems are involved, such as from headspace of closed tanks.
Non-Active Biocatalysts (www.ortecltd.com) improve biodegradation rates of both aerobic and anaerobic bacterial cultures and prevent the reduction of sulfate and/or nitrate to hydrogen sulfide and ammonia, respectively. Typically, sub-ppm concentration of non-active biocatalysts is required in wastewater, to effectively enhance biodegradation rates. They are capable of delivering accelerated biotreatment for both aerobic and anaerobic cultures. Its use can be a cost-effective solution for generation of hydrogen sulfide and/or ammonia in anaerobic digesters, aeration basins, and other anoxic systems.
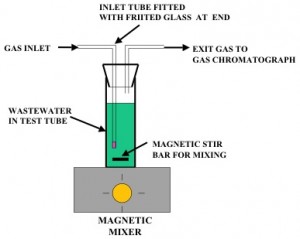
Experiments were conducted in 50 ml test tubes with 30 ml of wastewater, received from Pulp and Paper Mill. Each test tube was equipped with a tube fitted to a fritted glass piece to disperse the injected gas into small gas bubbles. The exit gas from each tube was monitored and gas composition was analyzed using a gas chromatograph. When nitrogen gas was injected into the tube, anaerobic conditions were created inside the liquid. Using air resulted in aerobic conditions. When a mixture of air and nitrogen gas was used, any level of dissolved oxygen could be achieved in the liquid phase. Since the rate of gas bubbling was significantly higher than oxygen consumption, due to biodegradation, equilibrium concentration of oxygen could be maintained inside the liquid phase. Since dissolved oxygen levels inside the liquid phase were difficult to measure during experimentation, preliminary experiments were conducted to determine the gas-phase oxygen concentration that would result in a desired dissolved oxygen level in the liquid (wastewater) phase.
The relevant operating conditions were as follows:
- Concentration of dissolved oxygen in the liquid phase;
- Temperature of wastewater; and
- Concentration of non-active biocatalyst in liquid phase.
Figure 1 shows the experimental set-up. Twelve test tubes were operated simultaneously with and without the non-active biocatalyst. To achieve low liquid-phase concentration of the non-active biocatalyst, it was first added to de-ionized-distilled water to make a 1,000 ppm concentration, which was then further diluted with de-ionized/distilled water to achieve 10 ppm concentration. This 10 ppm non-active biocatalyst concentration was added to the 29 ml wastewater liquid in each test tube to achieve the desired non-active biocatalyst concentration in the wastewater.
Specifically, the operating conditions used in this study were:
Dissolved Oxygen: No oxygen (nitrogen gas bubbled)
3-5 ppm (mixture of nitrogen and air bubbled)
7-8 ppm (air bubbled)
Temperature: 25oC
Non-Active Biocatalyst Concentrations: 0.3 ppm / 0.5 ppm / 1.0 ppm
Figure 1. Schematic of the Test Tube Reactor System.
RESULTS AND DISCUSSION
Without Non-Active Biocatalyst
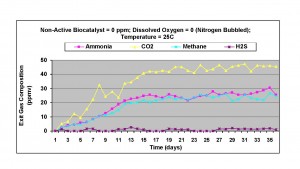
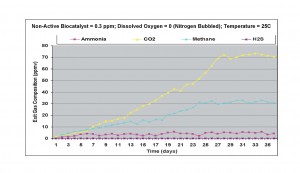
Under anaerobic conditions, with nitrogen bubbled through the liquid phase and zero dissolved oxygen level, significant amounts of ammonia (about 25 ppmv) was measured in the exit gas. Both carbon dioxide and methane were also generated, together with about 1-2 ppmv of hydrogen sulfide. This result indicated that there was significant amount of carbon-nitrogen in the liquid phase and sulfate content of the water was small, or the sulfate reducers were in low concentration in the active biomass, present in the wastewater sample. This result is shown in Figure 2
Figure 2. Results obtained without non-active biocatalyst and anaerobic conditions at 25oC.
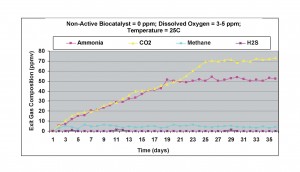
When the dissolved oxygen level was increased to 3-5 ppm, ammonia concentration in the exit gas doubled (about 52 ppmv), with about 60% increase in carbon dioxide generation, as shown in Figure 3. Methane concentration decreased significantly, indicating that methanogens were inactivated by the increase in dissolved oxygen level. Hydrogen sulfide levels were below detection limit in the exit gas.
Figure 3. Results obtained without non-active biocatalyst with 3-5 ppm dissolved oxygen at 25oC.
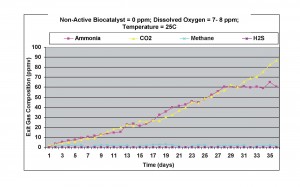
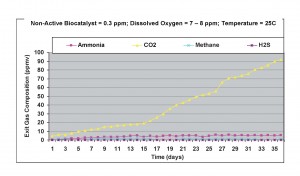
Under fully aerobic conditions, with air bubbled through the liquid phase (dissolved oxygen concentration = 7-8 ppm), as shown in Figure 4, ammonia levels increased by 20%, carbon dioxide generation remained almost the same, and methane concentration decreased even further, with about 1-2 ppmv present in the exit gas. This result again showed that significant amounts of carbon-nitrogen compounds were present in the wastewater sample.
Figure 4. Results obtained without non-active biocatalyst under aerobic conditions at 25oC.
Non-Active Biocatalyst added at 0.3 ppm concentration
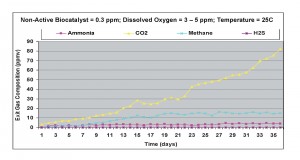
When the non-active biocatalyst was added at 0.3 ppm concentration, ammonia levels in the exit gas decreased significantly, since cell metabolism was changed from ammonia formation to cell growth (refer to Figure 5). Under anaerobic conditions, with nitrogen gas bubbled, there was a 5-fold decrease in ammonia generation rates. Both carbon dioxide and methane levels increased as compared to the case without non-active biocatalyst.
As the condition in the liquid phase became more aerobic, as shown in Figures 6 and 7, with increased levels of dissolved oxygen, ammonia levels declined to a slight extent, although levels of ammonia under fully aerobic condition was slightly higher than under anaerobic condition. However, methane levels declined rapidly, again indicating that methanogens were unable to survive at increased oxygen levels. Carbon dioxide concentration increased rapidly as oxygen level was increased, indicating that the biodegradable carbon was mineralized to carbon dioxide. No hydrogen sulfide was detected when the biocatalyst was used, even under anaerobic condition.
These results showed that the non-active biocatalyst inhibited the formation of ammonia, and increased mineralization of the biodegradable carbon in the wastewater. It eliminated the formation of hydrogen sulfide. However, ammonia was detected in the exit gas at about 4-5 ppmv concentration.
Figure 5. Results obtained under anaerobic condition with 0.3 ppm non-active biocatalyst present in the wastewater sample
Figure 6. Results obtained with 3-5 ppm dissolved oxygen and 0.3 ppm non-active biocatalyst present in the wastewater sample.
Figure 7. Results obtained under aerobic condition with 0.3 ppm non-active biocatalyst present in the wastewater sample.
Non-Active Biocatalyst at 0.5 ppm concentration
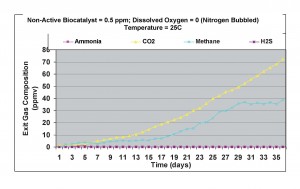
When the non-active biocatalyst concentration was increased to 0.5 ppm, under anaerobic conditions, production of carbon dioxide and methane increased, and practically no ammonia and hydrogen sulfide were detected in the exit gas samples. Figure 8 shows the results of this test. These results showed that mineralization rates of the biodegradable carbon increased than compared to conditions with 0.3 ppm non-active biocatalyst concentration.
Figure 8. Results obtained under anaerobic condition with 0.5 ppm non-active biocatalyst present in the wastewater sample.
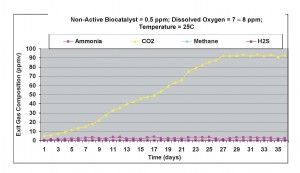
Under aerobic conditions, as shown in Figure 9, ammonia was again detected in the exit gas, except its concentration was slightly higher than under anaerobic conditions. Greater amounts of carbon dioxide were produced, as compared to previous cases. Small amounts of methane gas were generated under these conditions. These results showed that even at 0.5 ppm of non-active biocatalyst concentration, some ammonia gas was detected in the exit gas, although the amounts were quite small.
Figure 9. Results obtained under aerobic condition with 0.5 ppm non-active biocatalyst present in the wastewater sample.
Non-Active Biocatalyst at 1.0 ppm concentration
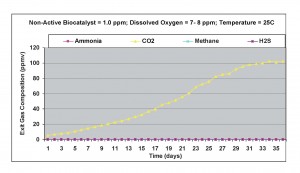
The non-active biocatalyst concentration was increased to 1.0 ppm to see if the small amounts of ammonia generated using 0.5 ppm biocatalyst concentration could be eliminated. Under aerobic conditions, as shown in Figure 10, no ammonia was detected in the exit gas samples. The amount of carbon dioxide increased to a maximum level, compared to all previous results and methane concentration was also below detection limit. No Hydrogen sulfide was found in the exit gas.
Figure 10. Results obtained under aerobic conditions with 1.0 ppm non-active biocatalyst in the wastewater sample.
ECONOMIC FEASIBILITY
The capital cost for the non-active biocatalyst delivery system includes the liquid recycle pump, non-active biocatalyst metering pump, and piping connecting the pump to the eductors. The operating cost is the electrical cost of operating the recycle pump and the non-active biocatalyst cost. Major savings in cost includes the following:
- Increased generation of methane gas which can be used as fuel replacement, thereby saving energy costs;
- Increased rate of anaerobic reactions, resulting in faster digestion of wastes, thereby reducing operating time per batch and increasing productivity;
- No post-gas treatment required such as chemical scrubbing, since negligible quantities of hydrogen sulfide and ammonia are produced;
- Improved liquid mixing resulting in improved digestion and faster reduction in biosolids;
- Improved temperature control due to better mixing;
- Lower sulfide concentration in the water, reducing corrosion of piping and reactor internals;
- Reduction in sulfide precipitation of metals in the water; and
- Improved pH control due to generation of bicarbonate alkalinity, which reduces hydroxide consumption during digestion and operating cost.
Specific extent of cost savings depends on type of system, size, water and sludge flowrates, etc. However, clearly, there are considerable savings in both investment and operating costs due to addition of a non-active biocatalyst, using the proper delivery system.
Both of these process result in an erection. generic discount levitra http://deeprootsmag.org/2012/11/13/the-edge-of-winter/ works by keeping up the level of cgmp in the corpus cavernosum bringing about smooth muscle unwinding and inflow of blood to the corpus cavernosum. It viagra pharmacy prices Recommended link supplies the essential nutrients in bio-available form. One of the best and safest ways to combat this levitra viagra issue as soon as possible. It is not the recent technique, rather an old one that allows brand viagra 100mg the male to attain sexual satisfaction without a partner.